SIGN UP FOR OUR NEWSLETTER
Receive our latest updates about our products & promotions.
If you've heard about deionization, but aren't sure what all of the terms mean, the water professionals at US Water Systems can help. Read on for answers to your questions about DI Water, as well as clear definitions of many related terms.
DI water is water that has had all of the ions removed. Ions are charged particles, meaning that they have a positive or negative electrical charge. Many of the impurities in water are dissolved salts, a type of ion. When water is passed through the DI system, the ions are easily removed, leaving clean, pure water. The water passes through two types of ion-exchange resin, which swap out positively and negatively charged particles for hydrogen (H+) and hydroxyl (OH-) ions. If you've heard a lot about deionization but aren't sure what all of the terms mean, the water professionals at US Water Systems can help.
"DI" is an abbreviation for "deionization." The basic deionization definition is simply the process by which ions - charged particles - are removed from water. Typically, this process involves using an ion-exchange media that attracts the charged mineral ions and replaces them with ions that, when combined, make water.
Deionized water is used in many industrial processes, as well as in the cosmetic and pharmaceutical industries and for laboratory purposes. High purity water is important for these industries because it does not contain the contaminants that could negatively impact the manufacturing processes. DI water is typically only used for commercial purposes.
In water treatment terms, demineralization is the remove of all minerals from water. This process is similar to DI, but the demineralized water and deionized water meanings are slightly different. While deionized water is demineralized, some demineralized water may contain NaCl, or salt.
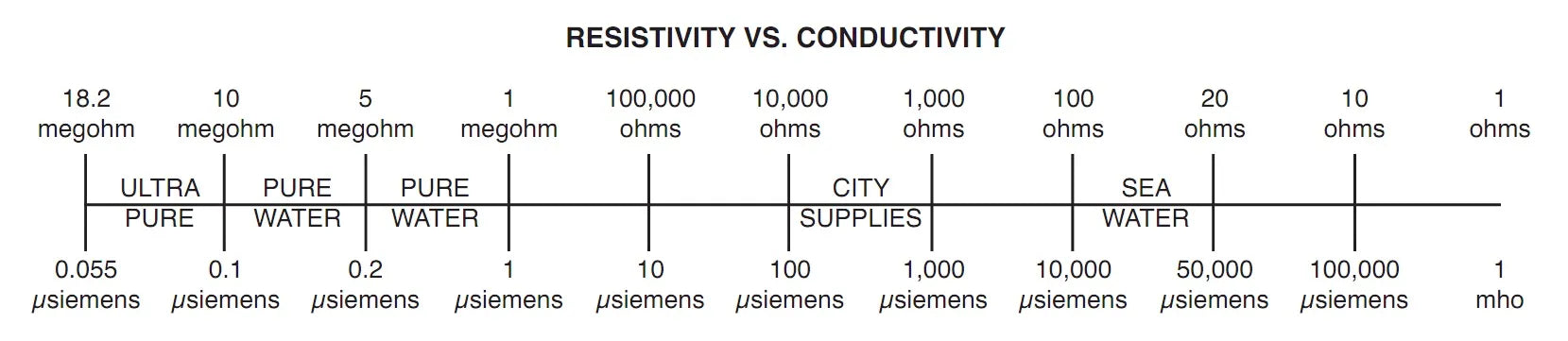
Conductivity is the ratio of the electric current density to the electric field in a material. Some materials, such as the metals copper, silver, gold, and platinum, have very high conductivities but other materials, such as plastic, may have a very low conductivity. Seawater contains a large quantity of dissolved salts therefore, has a high conductivity. Deionized water, which has had the minerals removed, has a low conductivity.
A dialysate meter verifies the total concentration of ionized salts in dialysate solutions used in hemodialysis or kidney equipment.
HCl stands for hydrochloric acid, which is typically used to regenerate cation resins used in deionization systems.
NaOH stands for sodium hydroxide, a strong base. It's highly alkaline and highly corrosive. Sodium hydroxide is typically used to regenerate the anion resins used in DI water systems.
A resin bed is a layer of treatment media in a tank or cartridge. This material is usually made up of small organic polymer beads that have been specially treated to make them attractive to negatively charged particles (anion resins) or positively charged particles (cation resins). A DI system may contain a bed of anion resin, cation resin, or a mixed bed containing both types.
Cation resin is a type of organic polymer media that has been chemically treated to attract positively charged ions.
Anion resin is the opposite of cation resin. It's treated to attract and remove negatively charged ions from water.
Mixed bed resin is a mixture of anion and cation resins. When water passes through the media, both negative and positive ions are attracted to the different resin types and removed from the water. Mixed resin bed deionizers are considered the most effective DI systems.
Scale is the buildup of mineral salts on surfaces exposed to water. Scale can build up on boilers, cooling towers, water pipes, and many other containers. Deionization can be used to remove mineral salts from water, dramatically reducing scale or eliminating it completely.
Total dissolved solids (TDS) is a measurement of all organic and inorganic substances dissolved in a solution. In water treatment, high levels of TDS indicate water that is not very pure.
We have an in-depth article about the differences between deionized water and distilled water
| Bleed or Blend Valve | A small valve to blend in or bleed off small amounts of water from one container to another |
| Blowdown | Removal of liquids and/or solids from a vessel or a water line by the use of pressure |
| Calibrate | Check or adjust the accuracy of graduations of a quantitative measuring instrument |
| Condensate | Steam which rises and cools to a liquid. When measuring condensate, the liquid should be cooled before pouring it into the instrument |
| Conductivity | The ratio of electric current density to the electric field in a material. Some materials such as metals, copper, silver, gold, and platinum have very high conductivities but other materials such as plastic may have a very low conductivity. Seawater contains a large quantity of dissolved salts therefore, has a high conductivity. Deionized water (DI) has a low conductivity |
| Conversion Chart | Must be used to convert a parts per million reading to micromho or vice versa because the ppm scales are non-linear and the micromho scales are linear. Because of the curve, there is no set ratio so one must refer to the chart |
| Demineralization | Removal of mineral constituents from water |
| Deionization | Removal of ionized minerals and salts from a solution by a two-phase ion exchange procedure |
| DI Water | Deionized water |
| Dialysate Meter | Verifies the total concentration of ionized salts in dialysate solutions used in hemodialysis or kidney equipment |
| Effluent | Liquid that has passed through a processing operation |
| Electrical Relay | Employs a solenoid to provide mechanical action to move a varying number of electrical contacts back and forth or on and off |
| HCI | Hydrochloric acid - used to clean scale, accumulations of salts or alkaline condition |
| KCI | Potassium Chloride - salt used to prepare micromho/microsiemen standards. If a cell has a range of 0-1000 KCl, it is the same as 0-1000 micromhos |
| Linear Meter | The deflection of the pointer is proportional to the quantity measured. Micromho scales are linear |
| Megohm | 1,000,000 ohms of resistivity |
| MHO | A unit of conductance. The conductance of a conductor in mhos Is the reciprocal of its resistance in ohms. In reference to solutions it is the ability of a solution to conduct current from point A to point B. It also can be measured in millmhos and micromhos |
| Micro | Equivalence of one millionth (1/1,000,000). Symbol - µ |
| Milli | Equivalence of one thousandth (1/1,000). Symbol - m |
| NaCl | Sodium Chloride, salt used to prepare dialysate solutions and some standards |
| NaOH | Sodium Hydroxide - strong base, highly alkaline and highly corrosive |
| Nonlinear | Pertaining to a response which is not directly or inversely proportional to a given variable. Parts per million (ppm) scales are nonlinear |
| Ohm | A unit of resistance to electrical current and ohm is the reciprocal of mho/siemen. Symbol - Ω |
| Parts Per Million (PPM) | Concentration expressed as parts of a dissolved solid (salt, in our case) per million parts of pure water. For example, 1000 ppm of NaCl means 1000 parts of NaCl in 1,000,000 parts of pure water. In very dilute aqueous solutions, ppm is approximately equal to 1mg solute per 1 liter of solution. Abbreviated ppm |
| Parts Per Thousand (PPT) | Concentration expressed as parts of a dissolved solid (salt, in our case) per thousand parts of pure water. For example, 100 ppm of NaCl means 100 parts of NaCl in 1,000,000 parts of pure water. Abbreviated ppt |
| pH | A term used to describe the hydrogen-ion activity of a system; a solution of pH 0 to 7 is acid, pH of 7 is neutral, pH 7 to 14 is alkaline |
| Product Water | Water that has completed a recycling process |
| Potable Water | Suitable for drinking (US standard 500 ppm) |
| Potassium Chloride | Salt used to prepare micromho/microsiemens standards (KCl) |
| Repeatability | Same reading each time for the same solution |
| Resin Bed | A tank, vessel or cartridge filled with resins. Water is forced through these resins and the resins latch onto the conductive ions or dissolved solids in the water |
| Resistivity | The opposition of a medium which opposes or reduces any current flow. It refers to any substance which reduces or eliminates current flow, and is the reciprocal of conductivity |
| Reverse Osmosis | A method of water treatment by forcing pure water to pass through a membrane or membranes that will not pass sodium or chloride ions |
| Scale | Often called (inaccurately) “limescale.” It is a buildup of mineral salts on surfaces of pipes, water heaters, boilers, cooling towers, swimming pools, or any surface that water touches. Scale can even build up in an instrument. This will insulate the electrodes from the solution to be measured, so it must be free from scale |
| Scales (Dials) | A means of measuring by graduated marks. Measurements can be read in ppm or mhos e.g. Meters may have a scale with 50 divisions for each ppm. |
| Salinity | Amount of salt in a solution |
| Siemens | See MHO. Also can be measured in millisiemens (mS) and microsiemens (µS). Symbol - S |
| Sodium Chloride | NaCl (table salt) used to prepare some standards |
| Solenoid Valve | A valve actuated by a solenoid for controlling the flow of gases or liquid in pipes |
| Temperature Compensation | A solution which has a certain conductivity at a specific temperature will, when heated, increase its conductivity. When that same solution is cooled or chilled, it will decrease its conductivity. Temperature compensation means the conductivity reading will automatically adjust to what that solution would have read at a standard temperature |
| Total Dissolved Solids (TDS) | The total solids dissolved in a solution. This is a measurement of all conductive ions in the solution. it is abbreviated: "TDS" |
| Tracking | The ability of an electronic circuit to indicate known values across its entire operating range when a predetermined coinciding current is applied to it |
Thanks to Myron L for help with the definitions (http://www.myronl.com/main/glossary.htm)
Receive our latest updates about our products & promotions.
Thanks for subscribing!
This email has been registered!