Why Water Filtration Is Important For Beer Brewers
Water is the main component of beer, and it has one of the greatest influences on the beer's flavor. Historically, brewers used the water locally available to brew their beer, leading to the distinct regional flavor profiles that exist globally. For instance, West Germany's Dortmund has water that is very hard. It is rich in calcium, sulfates, and chlorides. The small coal-mining town's golden pale lager is now internationally heralded for its distinguishing crisp, hoppy flavor, originally bestowed on it by the Dortmund water source.
Since ancient times brewmasters have been experimenting, developing distinctive and unique beer formulas. With experimentation, they quickly learned that the quality and make-up of their water could help cultivate unique flavor profiles for their beers. Water filtration systems like reverse osmosis have provided brewmasters with a solid starting point, from which they can express the creativity that allows their recipes to shine. Pure water establishes consistency across batches and can mimic the water quality of famed beer regions. Reverse osmosis water gives beer brewers extensive control over taste and the ability to innovate.
How water quality affects the taste of beer
Since beer is around 95% water, it is natural that the quality and mineral composition of the water has a large impact on the flavor. Two important elements of water will affect the taste of the beer: mineral composition, and pH. Water's hardness and mineral make-up will infuse flavor and greatly influence the fermentation process. The pH of the water fosters the rapid reproduction of yeast during the fermentation stage. Alkalinity can create a buffer to protect the pH from fluctuation throughout the brewing process, making sure bacteria is unable to grow and stripping excess tannins from the beer.
The Beer Brewing Process
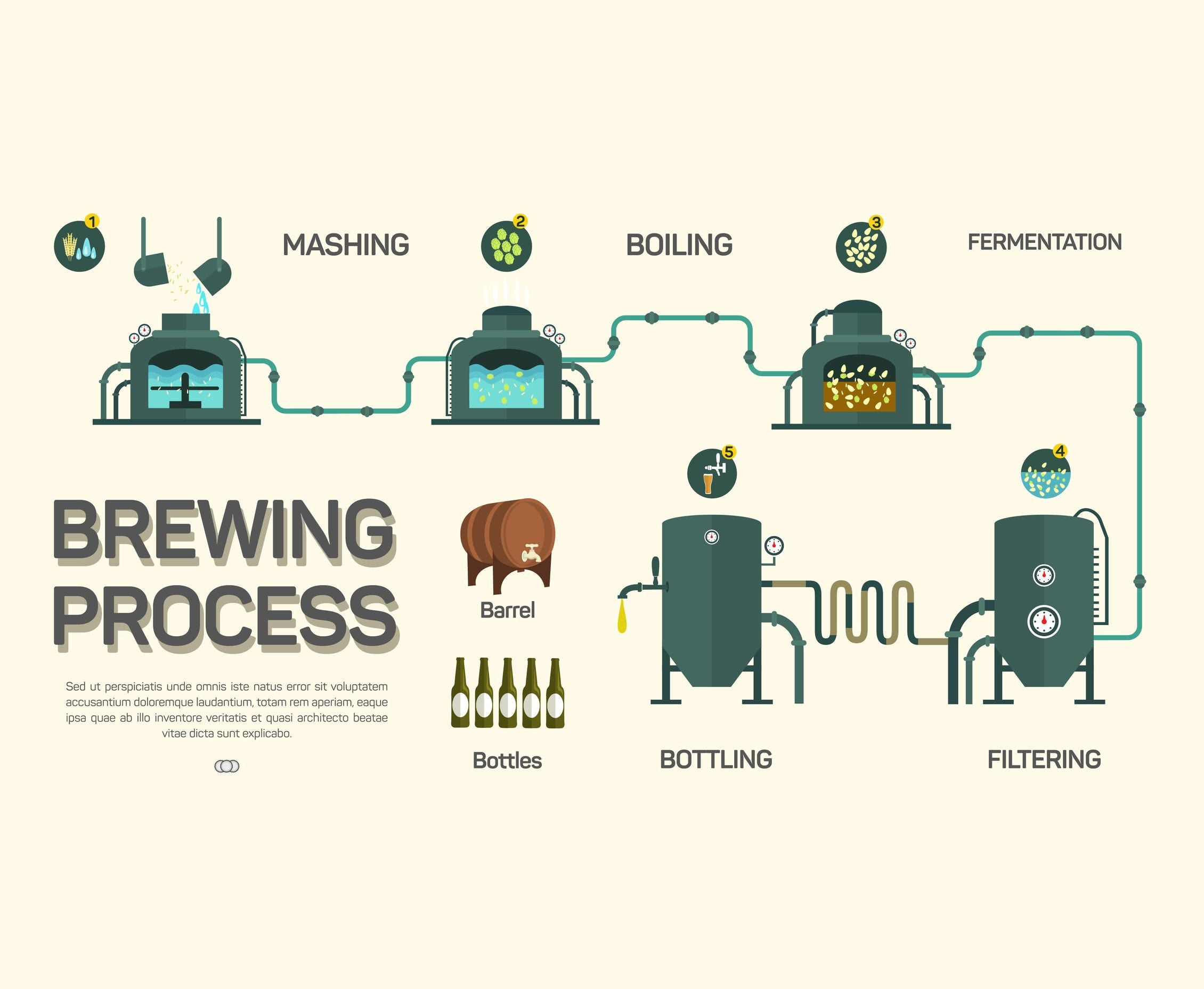

Mashing, also known as the hot water steeping process, initiates the brewing process and prepares the barley for fermentation. During mashing, crushed grains are hydrated with hot filtered water, which creates a thick mixture and activates enzymes in the malt. These enzymes convert soluble grain starches into fermentable sugars. This sugary liquid is called the wort, and it is a combination of proteins and carbohydrates that will influence the malt, mouthfeel, and body of the brew.
When the wort is ready to be fermented, it is anywhere between 80-90% water. Though the wort will extract flavors and aromas from the hops, it is only able to do so by being boiled in water and passing through a wort chiller. Water is essential to how these flavor profiles develop. The enzymatic action transpiring in the mash is entirely enabled by water and the mineral content of the water. Water can be considered the foundation for the process of brewing beer.
What Dissolved Minerals Affect the Taste of beer?
The minerals present in the water used for brewing will determine the flavor profile of the beer. Water hardness, which is the measurement of the (TDS) total levels of dissolved calcium and magnesium, imparts a strong hoppy flavor onto the beer.. If chloride is in the water, it gives the beer a rich mouthfeel. Sodium, sulfates, and carbonates all act to influence fermentation taste. The presence (or absence) of each dissolved solid will create a unique flavor characteristic during the brewing process. However, it is essential to use them deliberately, as too great an amount can cause unpleasant and overpowering tastes.
The mineral ions work in conjunction with the pH and alkalinity throughout the brewing process. Carbonates and bicarbonates stabilize the pH level during the mashing. If there is too much chloride present in the brewing water, the fermentation process can be hindered as chloride will destroy the yeast. The understanding of your water's composition is paramount, this is why the precision a brewmaster provides is integral to any brewery.
Good Ions In Beer Brewing Water:
Calcium (Ca+2): Calcium, a mineral found in high concentrations in regions where groundwater passes through rocks like limestone and gypsum, is one of the two minerals that make up water hardness. While hard water is a nuisance for a homeowner, it can greatly benefit a brewmaster. Calcium is popular among brewmasters because it lowers the pH levels, precipitates proteins, and is a yeast nutrient. Calcium has long been known to improve the clarity of the beer.
How to raise calcium levels in brewing water:
- Gypsum
- Calcium sulfate
- Calcium chloride
- Hydrated lime
Magnesium (Mg+2): Magnesium, the other component of water hardness, is of less importance to brewing, but remains a critical ion to have present. Magnesium can also be considered a yeast nutrient, and many brewmasters use it to ensure healthy yeast growth during the fermentation process. When used in moderation, magnesium accentuates the overall flavor of the beer, but too much of it will make the beer taste sour and acerbic.
How to raise magnesium levels in brewing water:
- Epsom salt (magnesium sulfate)
Sodium (Na+1): Sodium can accentuate the body and flavor of the beer, and add the desired sourness to beers. Too much sodium will cause the beer to have a metallic and harsh taste. Sodium has a very limited effect on the mash and flavor in low amounts. It can, however, be toxic to yeast and can prevent fermentation if used in too high of concentrations. As with most added minerals, your desired beer flavor will predetermine how much sodium you add.
Since high levels of sodium have such a dramatic impact on taste, you should never use softened water as brewing water. Through a process called ion exchange, water softeners exchange calcium and magnesium from the water by replacing them with sodium ions.
How to raise sodium levels in brewing water:
- Non-iodized salt or sea salt
- Baking soda (sodium bicarbonate)
Chloride (Cl-): Popular for the complex mouthfeel it lends beer, chloride is usually added to the water in either the form of calcium chloride or sodium chloride. They also promote stability during the mashing process. Chloride is often used in conjunction with sulfate to complement the bitterness of hoppier beers. The ratio of chlorides used is usually dependent on the sulfates, as the two produce opposite flavor profiles in the beer. Deliberate proportions of chloride and sulfate can balance out sweetness and hoppiness within a brew.
How to raise chloride levels in brewing water:
- Calcium chloride
- Sodium chloride
Sulfates (SO4-2): Sulfates bestow the beer with a dry, crispy taste, and as sulfates are alkaline, they can be used to mildly reduce the acidity of the mash. Where a bitterness is desired, sulfates can be used to bolster that flavor profile. The amount of sulfates added varies dramatically based on the desired levels of hoppiness. If the sulfate concentration is too high, it can react with the yeast and create hydrogen sulfide, which will make your brew reek of rotten eggs.
How to raise sulfate levels in brewing water:
- Calcium sulfate
- Gypsum
- Epsom salts (magnesium sulfate)
Carbonates & Bicarbonates (CO3-2/HCO3): Their strong alkalinity buffers the pH from becoming too acidic, resulting in astringent flavors and tannin-heavy beers. They also promote a round, malty taste in the beers, and at high levels can lend a bitterness desired by darker lagers. Since dark grains are more acidic, recipes for dark ales and lagers will usually require 100-300 mg/L of carbonate. Pale ales like IPAs will usually have carbonate levels of 25-50 mg/L. If carbonate levels are not adjusted during fermentation, however, they run the risk of keeping the beer's pH too high.
How to raise carbonates and bicarbonates in brewing water:
- Baking soda (calcium bicarbonate)
- Chalk
Ions That Can Ruin Beer Brewing Water:
Chlorine & Chloramines (Cl2): Chlorine is widely used by municipalities as a chemical disinfectant, protecting the city water supply from bacterial contamination. Unfortunately, chlorine is detrimental to brewing. It can be detected in beer in amounts as low as 5 parts per billion, chlorine leaves the beer with a palate-ruining plastic taste. When chlorine reacts with ammonia, it forms chloramine, a contaminant even trickier to eliminate from water. Not only do chloramines spoil the taste of the beer, they also prevent the yeast from properly fermenting the brew.
Iron (Fe+2 & Fe+3): Iron is never a desirable ion in beer. Since iron is the most common mineral found in the earth, many well owners are left with the undesirable results of iron-rich waters. Discoloration, dark orange and brown stains on fixtures, and destroyed appliances all result from even just 0.3 mg/L of iron in the water. Furthermore, iron gives the beer an inky aftertaste and interferes with proper fermentation. As little as 0.05 mg/L of iron in your water can ruin your brew.
Manganese (Mn+2): Any level of manganese greater than 0.01 mg/L is enough to leave your beer tasting metallic and unpleasant. Manganese ions, often found in combination with iron, will affect not just the flavor, but also the beer's clarity and threaten the health of the yeast during fermentation.
Nitrates/Nitrites (NO3- & NO2-2): Nitrates and nitrites can enter water through agricultural runoff, animal waste, and pollution. For well owners in farmlands, these contaminants can exist in high levels and present serious health problems if ingested. But, these contaminants are only regulated in water above 10 mg/L by the EPA, and any level above zero risks destroying the flavor of your beer.
How does pH affect beer brewing?


The pH of the brewing water has a strong influence on the taste of the beer and is critical to ensuring that enzymatic action transpires in the mash. The enzyme requires an acidic environment to properly convert the grain starches to fermentable sugars. Yeast thrives in lower pH, meaning the wort will have healthier fermentation. PH that is too high leads to the beer losing the complexity of its flavor, but if pH is too low you run the risk of bacteria can also developing during the fermentation.
Most water requires the appropriate mineral composition since it is neutral to alkaline by nature. Once again calcium plays a large role in promoting an acidic environment. Calcium is able to overcome the malt phosphates and the alkaline buffer. Carbonates and bicarbonates provide support to the alkalinity of the mash. This ensures that the pH does not fluctuate too wildly in either direction. The grains themselves are also acidic, which helps aid the enzymes in breaking down the starches. However, the pH of the mash will still need to be monitored throughout the brewing process, usually by an experienced brewmaster.
Lactic acid is a popular method that is used to lower the pH of the mash, as it does not add any disagreeable flavor to the mash. Phosphoric acid is found in many commercial beverage products, It has the advantage of imparting no taste. Phosphoric acid is less acidic than lactic acid, allowing for a larger margin of error when attempting pH adjustment. Buffers and stabilizers can also be added to the mash, which will achieve a target pH level by reacting with the phosphates in the malt. These will increase the calcium and magnesium content but will hold the target pH without fluctuation.
What are the best water filtration systems for brewing?
Reverse osmosis is known as the industry favorite for high-purity brewing water. RO systems eliminate over 96% of dissolved salts, solids, and minerals from the water. The RO's semi-permeable membrane reverts the water to a perfect baseline for brewers to move forward from. However, there are many options available to brewers seeking to improve their water quality and access a broader range of recipes. Catalytic carbon can remove chloramines from water, which will enhance your brewing water's taste. Finding the appropriate system for your home brewing needs is all that stands between you and the best batch of beer you've made.
Why Reverse Osmosis Is The Best Choice
At US Water Systems, we believe that craft beer brewing is an art, and that every artist deserves to have the highest quality canvas on which to ply their trade. For Beer brewing, that canvas is pure, contaminant-free water. The reverse osmosis process produces water that is mildly acidic and free of contaminants that affect the taste of your brew. This effectively provides the Brewmaster with water that is in a pure, blank state. Blank state water allows the Brewmaster to adjust levels of calcium, magnesium, sodium, chloride, sulfates, carbonates, and bicarbonates to deliver the desired flavor profile of the beer.
Got a favorite brew? Leave it in a mention below!







1 comment
Beer brewers require clean water to produce their product. A proper filtration process ensures a great taste, consistency, and quality for every brew. Cheers to that!
Leave a comment
Please note, comments need to be approved before they are published.